Auto Single Use Lancets by Lifescan for Fingerstick Sampling
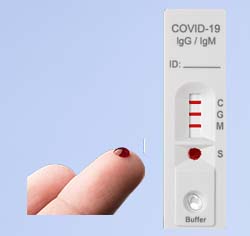
Rapid COVID-19 Antibody Test Kits with EUA for Fingerstick Samples
Rapid Cancer Early Screening Home Test Kits for Men and Women

Megna Health Rapid Antibody Test
Megna Health EUA rapid COVID-19 IgG/IgM test with fingerprick whole blood samples, CLIA waived.
Key Performance of the Rapid COVID-19 Antibody Test
Studied in 411 patients both positive and negative, data from the 541 patients including 130 patients with fingerstick samples are summarized in the Instruction for Use below
Also validated independently by NIH / NCI (National Cancer Institute) in 110 samples
NIH/National Cancer Institute Test Results:
Sensitivity: Combined IgM & IgG 100%
Sensitivity: IgG 100%
Simple and Easy to Perform
Just one drop of fingerstick sample and results in 15 minutes
Easy to Read & Interpret Results
| IgM + / IgG – | Recent infection with COVID-19 |
| Both IgM + / IgG + | Recent infection with COVID-19 |
| IgM – / IgG + | Previous infection with COVID-19 |
| Both IgM – / IgG – | No infection or not enough detectable antibodies in early infection |
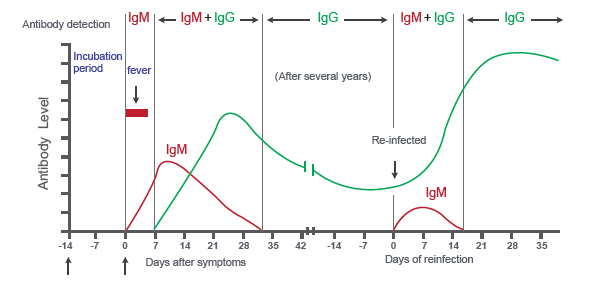
Results are for the detection of COVID-19 antibodies IgM and IgG. The IgM antibody to COVID-19 is generally detectable in blood a couple of days after initial infection, and IgG antibody is detectable typically after 7 days and stays in the immune system longer. Although the duration of time antibodies are present post-infection is not well characterized. Individuals may have a detectable virus present for several weeks following seroconversion.
Megan Health Rapid COVID-19 IgM/IgG Antibody Test – Instruction For Use
Megan Health Rapid COVID-19 IgM/IgG Antibody Test – Quick Reference Instruction For Use of Fingerstick
Megan Health Rapid COVID-19 IgM/IgG Antibody Test – Information for Health Professionals
Megan Health Rapid COVID-19 IgM/IgG Antibody Test – Information for Patients
Selected Customers


Early Cancer Screening Tests for Men
Early screening tests for the following cancers in men: prostate, liver, lung and colon cancers.
Early Cancer Screening Tests for Women
Early screening tests for the following cancers in women: ovarian, liver, lung and colon cancers.
Megna Rapid Antibody Test INTENDED USE
The Rapid COVID-19 IgM/IgG Antibody Combo Test Kit is a lateral flow immunoassay intended for qualitative detection and differentiation of Immunoglobulin M (IgM) and Immunoglobulin G (IgG) antibodies to SARS-CoV-2 in human serum, acid citrate dextrose (ACD) plasma and fingerstick whole blood. The Rapid COVID-19 IgM/IgG Combo Test Kit is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity. The Rapid COVID-19 IgM/IgG Combo Test Kit should not be used to diagnose acute SARS-CoV-2 infection.
Testing of fingerstick whole blood specimens is authorized for use at the Point of Care (POC), i.e., in inpatient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
Results are for the detection of SARS-CoV-2 antibodies. IgM and IgG antibodies to SARS-CoV-2 are generally detectable in the blood several days after initial infection, although the duration of time antibodies are present post-infection is not well characterized. Individuals may have a detectable virus present for several weeks following seroconversion.
Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities.
The sensitivity of the Rapid COVID-19 IgM/IgG Combo Test Kit after early infection is unknown. Negative results do not preclude acute SARS-CoV-2 infection. If acute infection is suspected, direct testing for SARS-CoV-2 is necessary.
False positive results for Rapid COVID-19 IgM/IgG Combo Test Kit may occur due to cross-reactivity from pre-existing antibodies or other possible causes. Due to the risk of false positive results, confirmation of positive results should be considered using second, different IgG or IgM assay.
The Rapid COVID-19 IgM/IgG Combo Test Kit is only for use under the Food and Drug Administration’s Emergency Use Authorization.
Frequent Asked Questions
Q: Why should I try the rapid antibody test?
A: Rapid antibody assay can provide you with information about one’s recent and previous infection. Combo antibody test kits like Megna Health’s kit test both IgM and IgG simultaneously. IgM forms 2 -3 days after symptoms onset and lasts about 1 week, while IgG forms about a week after symptoms onset and lasts longer. IgG is an indication of immunity to some extent. Antibody tests are not considered diagnostic purpose for COVID-19, it provides additional information that other types of tests doesn’t.
Q: Is the rapid antibody test safe?
A: Yes. It operates with fingerstick whole blood samples, serum, and plasma (ACD) samples. Megna Health is currently seeking authorization for prescription home use with fingerstick samples.
Q. What’s the difference between PCR and antibody tests?
A: PCR tests the presence of the virus, which mainly happens at the beginning of the infection, while antibody tests for the presence of IgM (2 -3 days after infection ) and IgG (one week after infection). But the main advantage of rapid antibody tests is easy sampling and quick results (less than 15 minutes), and no need for expensive PCR equipment.
Q: Is Megna Health’s Rapid Antibody Test authorized by FDA?
A: Yes, it is authorized by FDA EUA (Emergency Use Authorization) in July 2020 and extended to CLIA-waived Point of Care facilities with fingerstick samples on April 22, 2021. Attached is the FDA approval cover letter
Megna Health Rapid COVID-19 Antibody Combo Test Kit FDA EUA Approval
Q: Is the product manufactured in the US?
A: Yes. Our test kits are manufactured in Pennsylvania.
Q: What’s Megna Health’s Rapid Antibody Test’s accuracy?
A: Based on an independent study of 110 subjects by NIH / NCI (National Cancer Research), our test kits demonstrated 100% sensitivity for IgG, and for combined IgG/IgM, specificity has been shown to be 95%.
Q: How long does the rapid test take to see the results?
A: 15 minutes
Q: How much sample does the assay take?
A: Just one drop from fingerstick sampling.
Q: What’s the difference between IgM, IgG alone vs. the combo test?
A: There are products just for IgG or IgM, but combo test kits test both IgG and IgM from the same test. As discussed earlier, IgM antibodies indicate current infection, it would be a complement to PCR and antigen assays. However, since IgM antibodies last only a limited time before IgG formation, it’s technically more challenging to test IgM with acceptable sensitivity compared to just detecting just IgG antibodies. Therefore, combo test provides more timely information about recent or past infections.
Q: Who can perform the test?
A: Megna Health Rapid COVID-19 Test can be performed with fingerstick samples at any CLIA waived Point of Care facilities such as clinics, dentist’s offices, pharmacies, etc, and any CLIA-certified laboratories,
Q: What’s the next product goal for Megna Health’s development?
A: Megna Health has submitted an application for an extension to prescription home use for its Rapid COVID-19 Test Kit.
Q: What info can the antibody test help with the vaccine process?
A: Be aware that SARS-CoV-2 antibody tests help healthcare providers identify whether someone has antibodies to SARS-CoV-2, the virus that causes COVID-19, indicating a prior infection with the virus. However, more research is needed to understand the meaning of a positive or negative antibody test, beyond the presence or absence of antibodies, including in people who received a COVID-19 vaccination, in people who have been exposed and have SARS-CoV-2 antibodies, and in people who are not fully vaccinated.
If you have not been vaccinated: Be aware that a positive result from an antibody test does not mean you have a specific amount of immunity or protection from SARS-CoV-2 infection. If you have a positive test result on a SARS-CoV-2 antibody test, it means that it is possible you were previously infected with the SARS-CoV-2 virus. Talk with your healthcare provider about the meaning of your SARS-CoV-2 antibody test results.
If you received a COVID-19 vaccination: Continue to follow the CDC’s recommendations for fully vaccinated people. Be aware that if you have a positive test result on a SARS-CoV-2 antibody test, it is possible you were previously infected with SARS-CoV-2. A COVID-19 vaccination may also cause a positive antibody test result for some but not all antibody tests. You should not interpret the results of your SARS-CoV-2 antibody test as an indication of a specific level of immunity or protection from SARS-CoV-2 infection. Talk to your healthcare provider or your state and local health departments if you have questions about whether an antibody test is right for you.
Q: Is the antibody test covered by my insurance?
A: Yes. HCPT codes are well established for reimbursement with a prescription from a doctor.
Rapid Antibody Test PRINCIPLE
Rapid COVID-19 IgM/IgG Combo Test Kit utilizes the principle of immuno-chromatography. Mouse anti-human IgM and human IgG antibodies are immobilized on the nitrocellulose membrane respectively, as two individual test lines (IgM line and IgG line) in the test window of the test device. The IgM line in the test window is closer to the sample well followed by IgG line. As the test sample flows through the membrane within the test device, the colored COVID-19 virus recombinant antigen-colloidal gold conjugate forms complexes with specific antibodies (IgM and/or IgG) to COVID-19 virus, if present in the sample. This complex moves further on the membrane to the test region where it is captured by the anti-human IgM and/or human IgG antibodies coated on the membrane leading to the formation of a colored band, which indicates positive test results. The absence of this colored band in the test window indicates a negative test result. A built-in control line will always appear in the test window when the test is performed properly, regardless of the presence or absence of anti-2019 novel coronavirus antibodies in the specimen.
Rapid Antibody Test WARNINGS AND PRECAUTIONS
For use under an Emergency Use Authorization Only. For in vitro diagnostic use only.
This test has been authorized by FDA under a EUA for use by CLIA waived Point of Care facilities and laboratories certified under CLIA, that meet requirements to perform moderate or high complexity tests.
This test has been authorized only for the presence of IgM and IgG antibodies against SARS-CoV-2, not for any other viruses or pathogens.
This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Do not use the product beyond the expiration date. Do not use the product if the pouch is damaged or the seal is broken. Handle all specimens as potentially infectious.
Follow standard Lab procedures and biosafety guidelines for handling and disposal of potentially infectious material. When the assay procedure is completed, dispose of specimens after autoclaving at 121º C for at least 20 min or treating with 0.5% Sodium Hypochlorite for 1-2 hours. Tests are for single use only.
Rapid Antibody Test CONDITIONS
The Rapid COVID-19 IgM/IgG Antibody Combo Test Kit Letter of Authorization, along with the authorized Fact Sheet for Healthcare Providers, the authorized Fact Sheet for Recipients, and authorized labeling are available on the FDA website:
https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas
Authorized laboratories using the Megna Health Rapid COVID-19 IgM/IgG Antibody Combo Test Kit in the conditions above), must adhere to the Conditions of Authorization indicated in the Letter of Authorization as listed above.
Authorized laboratories using Megna Health Rapid COVID-19 IgM/IgG Antibody Test will have a process in place for reporting test results to healthcare providers and relevant public health authorities, as appropriate.
